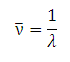Infrared radiation has lower energy than visible light. IR radiation has frequencies between, approximately, 4.3 × 10
14 to 1.0 × 10
12 Hz corresponding to wavelengths of 7.0 × 10
-7 m and 3.0 × 10
-4 m. As these numbers are hard to write and difficult to remember and work with, neither a frequency nor wavelength scale is commonly used. Instead, spectroscopists tend to use "wavenumbers" for IR spectroscopy. A wavenumber is the inverse of the wavelength,
λ, in cm:
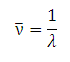
|
|
It has units of 1/cm or cm-1. It is directly proportional to the frequency and the energy of the radiation: IR radiation with a high wavenumber has higher frequency and energy than IR radiation with a lower wavenumber. Because wavenumber and frequency are directly proportional to one another, it is common for the two terms to be used almost interchangeably: even if IR frequencies are being discussed, it is important to look at the units as these may be wavenumbers!
The IR region that is of most use in spectroscopy falls between around 400 - 4000 cm-1.
|
In a typical
IR spectrometer, the sample is exposed to IR radiation in this range. When the energy matches that of a vibration (and that vibration leads to a change in the dipole
*), absorption occurs. Otherwise, the radiation passes through and no absorption occurs. A typical IR spectrum is shown below. The wavenumber of the radiation is plotted on the
x-axis. The fraction of the radiation that is absorbed - the
transmittance - at each wavenumber is plotted on the
y-axis. At wavenumbers where no absorprion occurs, the transmittance is 100%. An absorption corresponds to a '
trough' in the curve. If all the radiation is absorbed, the transmittance would be zero. The spectrum thus consists of a curve which is near 100% except for a number of troughs. It is these troughs, but commonly called 'peaks' confusingly, that show where the molecule is absorbing and hence the wavenumbers of its vibrations.
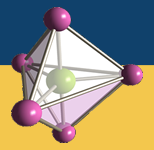
The methane molecule absorbs at 2 different energies. The IR spectrum of methane is shown below.
Click on each absorptions to see which corresponds to each vibration. You can
rotate the molecule using your mouse.